Both light and matter consists of tiny particles which have wavelike properties associated with them. Light is composed of particles called photons, and matter is composed of particles called electrons, protons, neutrons. It's only when the mass of a particle gets small enough that its wavelike properties show up.
To help understand all this let's look at how light behaves as a wave and as a particle.

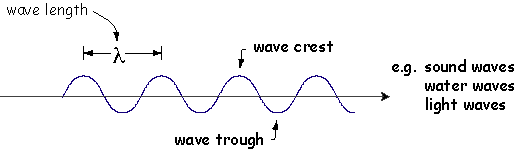
One behavior of waves is Diffraction
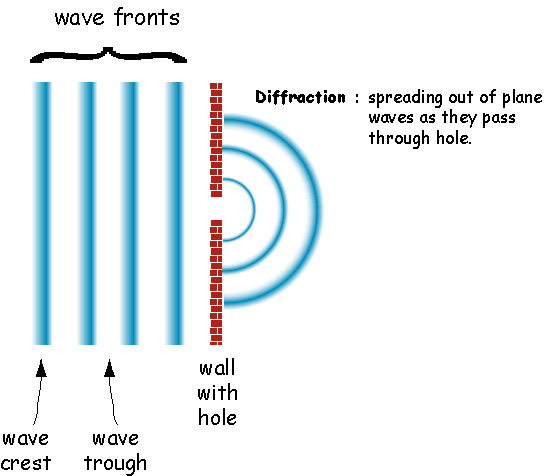
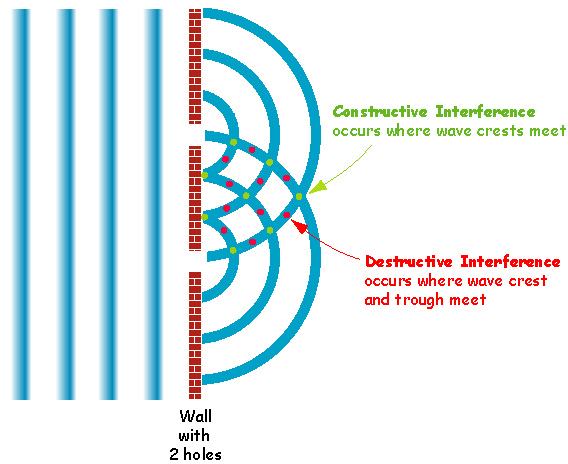
Another behavior of waves is
Interference
Although light is composed of
particles called photons, one can easily show that light can
be thought of as an electromagnetic wave that travels at the speed of
light.
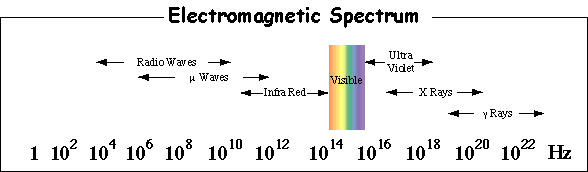
The different regions of the
electromagnetic spectrum are given different names. Below are the
names given to the different regions (frequency ranges) of light
according to their frequency range.
At this point you may think that it's pretty obvious that light behaves like a wave. But where's the proof that light is really composed of particles called photons? The proof comes from an experiment that is called the photoelectric effect.
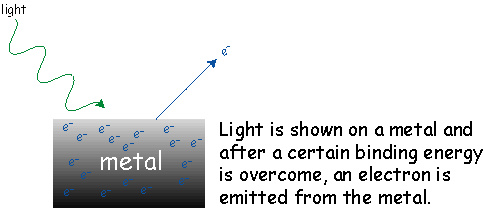
An important feature of this experiment is that the electron is emitted from the metal with a specific kinetic energy (i.e. a specific speed).
Now anyone who is familiar with the behavior of waves knows that the energy associated with a wave is related to its amplitude or intensity. For example, at the ocean the bigger the wave, the higher the energy associated with the wave. It's not the small waves that knock you over it's the big waves! So everyone who thought light is just a wave was really confused when the intensity of the light was increased (brighter light) and the kinetic energy of the emitted electron did not change. What happens is that as you make the light brighter more electrons are emitted but all have the same kinetic energy.
Well, they thought the kinetic energy of the emitted electron must depend on something. So they varied the frequency of the light and this changed the kinetic energy of the emitted electron.
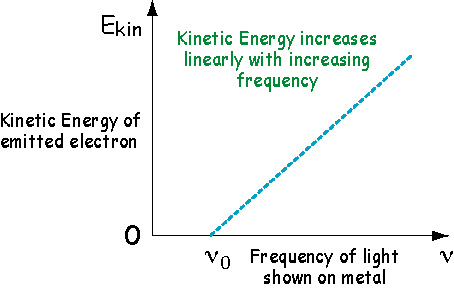
However, there is a critical frequency for each
metal, ![]() , below
which no electrons are emitted. This tells us that the kinetic energy
is equal to the frequency of the light times a constant (i.e., the
slope of the line). That constant is called Plank's Constant
and is given the symbol h.
, below
which no electrons are emitted. This tells us that the kinetic energy
is equal to the frequency of the light times a constant (i.e., the
slope of the line). That constant is called Plank's Constant
and is given the symbol h.
Now we can write an equation for the kinetic energy of the emitted electron.
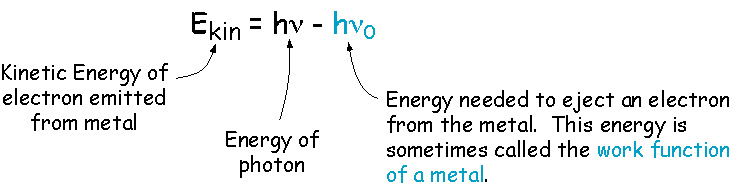
This result is not consistent with the picture of light as a wave. An explanation that is consistent with this picture is that light comes in discrete packages, called photons, and each photon must have enough energy to eject a single electron. Otherwise, nothing happens. So, the energy of a single photon is:
When this was first understood, it was a very startling result. It was Albert Einstein who first explained the photoelectric effect and he received the Nobel Prize in Physics for this work.
So, in summary-light is a particle, but has some wave-like behavior.