Temperature is a macroscopic property of matter -- that is, its effects can be described entirely in terms of objects that are big enough that you can see them. We can measure temperature with a thermometer and study its relationship to energy without any discussion of the nature of matter on the small scale.
We are told that matter is composed of small particles -- atoms and molecules. We should be reluctant to make use of any explanation of macroscopic phenomena that refers to properties of atoms and molecules, because you and I have never seen these atoms. It is easy to go wrong in a description of the behavior of matter that starts from an atomic description, because the behavior of a collection of atoms differs in important respects from our experience with particles -- for example, marbles do not continually fly about the room as do the atoms in a gas. Therefore we should avoid atomistic explanations of natural phenomena whenever possible.
However, there are some aspects of the behavior of matter that can be explained neatly in terms of the atomic model, and many expositions of temperature and heat and the properties of matter refer to the atomic model. The great physicist Richard Feynman once said that the most important thing science had ever discovered is that everything is made of atoms. Atoms are real, even if we can't see them!
Atoms
In the present context, in which we only wish to understand the behavior of matter under ordinary conditions, we do not need to know what is inside atoms, and we can think of them as small hard spheres that will stick together to form molecules. The rules that describe what will stick to what are the subject of the field of chemistry, but we should mention here the first triumph of this theory, which was the ability to explain the relationship between the amounts of chemicals that participate in a reaction: for example, how much air should be combined with how much gasoline to get complete combustion.
Temperature
Temperature has a very definite meaning at the atomic scale. At any temperature the atoms are wiggling back and forth like a classroom of third graders, and the temperature is directly a measure of the average kinetic energy of an atom. Please note:
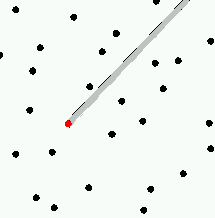
The molecules of a gas are
 |
| A gas. The red molecule might travel quite a ways (along the gray path) before it encountered another molecule. |
|---|
The atoms and molecules in liquids and solids are so close together that they are frequently in contact. You can get a sense of the hardness of atoms if you try to squeeze a stone. In a solid, each atom is walled in by its neighbors, so that none can change positions (you might think of a stadium parking lot, after a big game) In liquids there is just enough motion that molecules can move past each other (think of the pedestrian crowd leaving the stadium -- elbow-to-elbow, yet the stream of people somehow moves around obstacles).
Thermal expansion occurs on a scale that we can see, but it arises because of changes in the motion of atoms that we
cannot see. As a material heats up, the atoms move more.
Even the closely packed atoms
in solids move. (It's this atomic motion that transmits the heat we feel when grasping a hot pan from the oven.) As atoms move
more, they bump into their neighbors more and push each other away more. They move apart, and the result is thermal
expansion. The atoms stay the same, but the object gets bigger.
Check the box when you are done:
Discussion of thermal expansion