Boiling
At 100o C, water boils at atmospheric pressure. This
means that the liquid is able to turn into vapor and
the air gets pushed away.
 If we attempt
to raise the temperature above 100oC, the liquid water turns
into water vapor as fast as energy is added: the water is boiling and
the temperature does not change at all.
At high elevations the air pressure is less, and water boils at
a lower temperature.
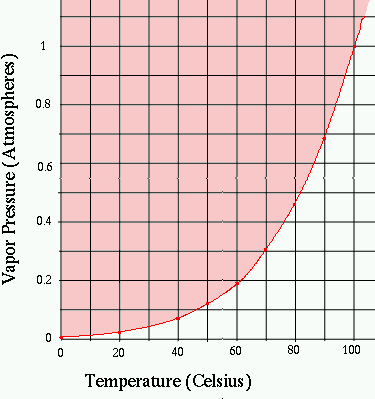
The graph shows how the boiling temperature varies with pressure:
for example, if the pressure is 70% of normal atmospheric pressure,
the boiling temperature is reduced to 90oC.
In the pink region above the curve, liquid
water can exist (we are below the boiling temperature), while in the
white region, we have only water vapor.
If we attempt
to raise the temperature above 100oC, the liquid water turns
into water vapor as fast as energy is added: the water is boiling and
the temperature does not change at all.
At high elevations the air pressure is less, and water boils at
a lower temperature.
The graph shows how the boiling temperature varies with pressure:
for example, if the pressure is 70% of normal atmospheric pressure,
the boiling temperature is reduced to 90oC.
In the pink region above the curve, liquid
water can exist (we are below the boiling temperature), while in the
white region, we have only water vapor.
This graph is closely related to the one on the previous
page. "Vapor pressure" is proportional to the amount of water
in the air, with a scale that varies a little with temperature.
Check the box when you are done: ![]()
What have we learned about changes of phase?
 If we attempt
to raise the temperature above 100oC, the liquid water turns
into water vapor as fast as energy is added: the water is boiling and
the temperature does not change at all.
At high elevations the air pressure is less, and water boils at
a lower temperature.
The graph shows how the boiling temperature varies with pressure:
for example, if the pressure is 70% of normal atmospheric pressure,
the boiling temperature is reduced to 90oC.
In the pink region above the curve, liquid
water can exist (we are below the boiling temperature), while in the
white region, we have only water vapor.
If we attempt
to raise the temperature above 100oC, the liquid water turns
into water vapor as fast as energy is added: the water is boiling and
the temperature does not change at all.
At high elevations the air pressure is less, and water boils at
a lower temperature.
The graph shows how the boiling temperature varies with pressure:
for example, if the pressure is 70% of normal atmospheric pressure,
the boiling temperature is reduced to 90oC.
In the pink region above the curve, liquid
water can exist (we are below the boiling temperature), while in the
white region, we have only water vapor.