A light bulb is basically a hot wire, and it emits not only visible light but a very large amount of invisible infrared light. A LED, in contrast, emits only light of a narrow range of wavelengths -- red, for example. This makes them much more efficient; they are beginning to show up in stoplights and car taillights, and other applications where we want light of just one color.
A light-emitting diode is related to a transistor. It turns electrical energy into light energy directly, without anything getting hot; in fact, they come close to the ideal of turning all of the electrical energy input into visible light. Their sensitivity to the direction of the current is inherent to the way they work; when you connect them to a battery the wrong way, no current flows and nothing happens at all. The bidirectional L.E.Ds (that give different colors for different current direction) are actually two diodes in one container.
The electrical behavior of a light emitting diode is not simple. It will not do anything at all unless the voltage is more than 1.6 V (for the red) or 1.9 V (for the green). (With a fresh D cell -- the standard flashlight battery -- you may get the faintest red glow). In contrast, an incandescent light bulb continues to conduct a current with low voltage, and is still glowing, but very dim and red.
Here's a brief description of how a light emitting diode works. They are
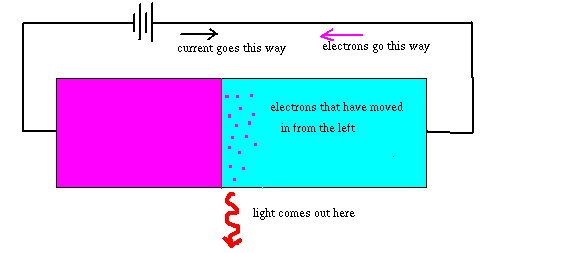
contain two special materials, joined to make one piece, as shown in the diagram:

One of the materials in the diode contains lots of free electrons, while the other doesn't -- instead it carries current in a different way. When we run a current through the diode, electrons leave the material where they live and move into the material where they are not supposed to be. They are at high energy as they move in, and now there is a low energy place for them to go to. So they suddenly fall into these electron-sinks. In the process, they have to get rid of the energy, and do so by making light.
Here is another diagram, that compares the diode to a waterfall.
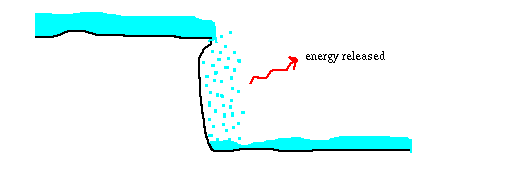
Water comes to the waterfall at a high elevation (so that it has high potential energy). As it goes over the waterfall, now there is a low energy place for the water to be, and the gravitational energy gets turned into other forms -- in real waterfalls, it is in the rapid motion of the water below the falls, and eventually warms the water up a little bit. But imagine that instead, each drop of water gives a scream as it falls over the cliff. That corresponds to the light that the electrons give off as they go over the electrical cliff in the diode.
It is an interesting fact (for which Einstein got the Nobel Prize) that light carries its energy in discrete packages (called quanta). Instead of a continuous stream of energy, it arrives like a rainstorm, in lumps or drops. Furthermore (again due to Einstein), red light has slightly smaller lumps of energy than blue light. To make one red quantum, the falling electron has to have the right amount of energy. If the cliff were higher, it would make a blue quantum instead. We also have to pump the electrons up to the top of the cliff (that is what the battery does), and so we have to have the appropriate voltage that matches the height of the electrical cliff. So nothing at all happens if the voltage is too low; right about 1.76 V the red LED turns on; a higher voltage doesn't accomplish much except to increase the current a bit (so the LED gets brighter). A green LED would need a slightly higher voltage (closer to 2 V).
When you connect the red LED to the battery the wrong way, you are trying to makehe electrons go the wrong way. Instead of moving into the blue material, they are trying to move away from it. But there aren't any electrons in the blue material to replace them, and so no current can flow. Nothing happens at all.
The section on the concepts of electricity