- 4 brass washers *
- 4 zinc-plated steel washers *
- a piece of aluminum foil (5 cm square) *
- 4 paper squares just wider than the washers *
- small dropper bottle of salty vinegar *
- buzzer * It's useful to connect a black clip-end wire to the
black wire from the buzzer, and a red clip-end wire to the red
wire.
- light-emitting diode

Making a cell:
-
Place a paper square on top of a zinc/steel washer.
-
Put one or two drops of the salty vinegar on the paper, over the
washer. We want to get the paper damp but not dripping.
-
Place a brass washer on the paper.
-
Push down on the washer. If any liquid oozes out, wipe it off (and
use less, next time). The brass top and the zinc bottom should not
have any liquid on them.
This makes a 0.7 volt cell. The zinc bottom is the negative end
and the brass top is the positive end.
-
Make three more of these brass/paper/zinc cells.
A single cell of this kind is not strong enough to cause
anything to happen with any of our electrical devices. However,
a stack of cells -- a battery -- will be able to do something interesting.
Build the stack on the piece of aluminum foil. This will give
a convenient way to provide an electrical
connection to the bottom of the stack, which is going to be rather wobbly.
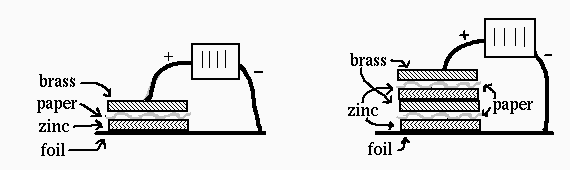
- Build a tower of two of the cells on the aluminum foil, as
shown. Always place them so that the brass washer is on top.
Putting the two cells together makes a battery. The voltage between
the aluminum foil and the top brass washer is 1.4 V.
It probably also will not do anything, but you could try to run the
buzzer with it by connecting the negative (black) wire to the
aluminum foil, and touching the positive (red) wire to the top
brass washer.
- Continue to build the battery by adding another cell
(zinc/moistened paper/brass). Check it with both the buzzer and the
light emitting diode (connect one of the light emitting diode wires to the
aluminum foil, and connect the other to the top of the stack).
- Then add the last cell, and check it again.
It is important that the bottom side of the zinc washers stay dry, that
the paper is damp, and that everything is in the right order.
You will notice that the battery works better if you disconnect
it and let it rest for a few seconds. It may also help to push
straight down gently, to improve the contact between the
washers.
When you are through studying the battery, take it apart and
wash and dry the washers. As you do so, notice that the paper has
become stained, and the brass washer discolored. These are evidence
of the chemical reactions that released the energy that the battery
was producing. If you run the battery long enough, you will remove
the zinc coating from the washer altogether.



