 A D cell (139 g) will deliver about 63,000 J
A D cell (139 g) will deliver about 63,000 JA C cell (69 g) will deliver about 33,000 J
An AA cell (24 g) will deliver about 11,000 J
An AAA cell (11 g) will deliver about 5,000 J
Units of energy and units of power
Comparing different sources of energy and power gets confused because the measurements are done in different units. Think how hard it would be to compare one kind of soap with another, if one kind was priced in pesetas, another in lira, and a third in rubles! But that is what the energy marketplace looks like. This comes about partly because the different forms of energy require different kinds of measurement technique, and partly because the energy suppliers are trying to use energy units that are of an appropriate size for the typical application (for example, you buy "10 gallons of gasoline" and not "1,350,000,000 Joules of gasoline").
More about batteries
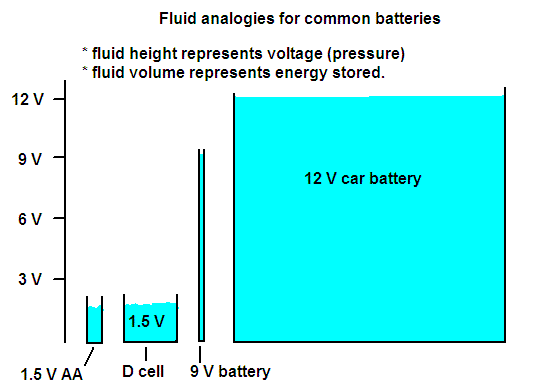
Batteries come in many sizes, and we use many different voltage levels. How did this come about?
The standard flashlight battery is about 1.5 V. This voltage is established by the choice of chemical reaction that is used. Batteries having this voltage have a very simple "single cell" internal construction -- the bottom and sides are one conducting piece and reactions of one kind take place at the inside surface; the top and a central post inside are another conducting piece where a different chemical reaction takes place; and the region between these is filled with a paste of chemicals that participate in the chemical reactions or keep the two reaction regions separate. The different kinds of batteries (Alkaline, Nicad, mercury, lithium, zinc-air, ...) have different reactions and slightly different voltages.
A battery is a portable source of energy. The amount of stored
energy depends on the chemical reaction that is involved and on how
much of the reacting chemicals are in the battery. The difference
between AAA batteries, AA batteries, C batteries, and D batteries
is that they store different amounts of energy. For example,
one
brand of batteries claims that its batteries perform as
follows:
 A D cell (139 g) will deliver about 63,000 J
A D cell (139 g) will deliver about 63,000 J
A C cell (69 g) will deliver about 33,000 J
An AA cell (24 g) will deliver about 11,000 J
An AAA cell (11 g) will deliver about 5,000 J
Single-cell batteries with higher voltages do not exist because they would contain dangerously reactive materials. The 9 V batteries used in portable radios and the 12 V batteries used in automobiles are not single cells -- they are many cells connected in series. Making a higher voltage this way does not increase the amount of energy that is stored, but makes it possible to get the energy out faster (i.e. it is useful where high power is needed).
Around the house we sometimes use low voltages (6 V or 12 V for doorbell circuits or some kinds of exterior lighting), especially where electrical safety is a concern. The standard house circuit is 110 V to 120 V, but with a simple rewiring can provide 220 V to run large airconditioners, stoves, and other kinds of heaters. The distribution lines within a city use several 1000 V, while power is moved from the power plant to the city by transmission lines that are 100,000 V or more.
Is it 110 V or 115 V or 120 V?
You will see all three values quoted. The power company tries to maintain 120V, but are allowed to be off by 5% either way, so the line voltage might be as low as 114V or as high as 126V.
There is a second complication: the voltage is AC, and is varying smoothly up and down.
The value that is quoted is the equivalent DC voltage that would make a light bulb just as
bright. Since the AC voltage is small for part of the cycle (while the current is reversing),
it has to be bigger than the equivalent DC at other times. Then "120 V AC" really means
"oscillating between +170 V and -170V"!
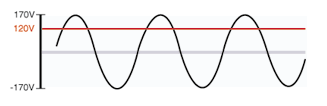
The dark line represents how the actual voltage varies with
time, and the red line is the DC voltage that would
make a light bulb just as bright.
The section on the sources of electrical energy