Joe's answer
There are two ways that I can think of that are used to measure the temperature
of the sun and other stars:
*The color of the light given off by a hot object is an indication of its temperature. A red hot object is cooler than a white hot object, for example. With a good measurement of the brightness of the sun for different wavelengths of light (which means roughly, for different colors of light) we can estimate the temperature of the surface of the sun.
*We can tell which atoms are in the atmosphere of the sun using a spectroscope. This also reveals how many atoms are excited. It has to be really hot to excite an atom, and by looking at which kinds of atoms are excited and which are not, we get a measurement of the temperature of the "atmosphere" of the sun.
However, this does not determine the temperature inside the sun. Astronomers have theories for this that they are very certain of, because there is an indirect test of it: the sun makes its power by nuclear reactions, and the center of the sun has to be a certain temperature for these reactions to take place.
My Question:
If I take a ray of sunlight and put it through a prism
and project the spectrum on a wall. Would a
very sensitive thermometer find that the red is warmer than the violet?
I think it would be, because the red is nearer to the infrared part of the spectrum.
Sally says
There are two standard ways to represent a spectrum. One shows how
much power arrives in each wavelength. The other shows how much power arrives
in each photon energy or frequency. Since wavelength is the reciprocal of
frequency, the two ways don't look very much alike.
However, the spectrum of a cooler object, like an incandescent
light bulb, has relatively less visible light, and the warmest part
of the spectrum would indeed be in the infrared (this is how infrared
light was discovered!).
My Question:
If
something that is white reflects all light, then why doesn't it (the white
object), rather than something that is black, make the area around it
hotter?
Joe's answer
*
Light carries energy
So when the sun is out, a dark object is going to be hotter than everything
else,
because it absorbs the light and gains the energy. A parking lot in July can
get really hot! Not only does it make you hot when you touch it, but it heats
up the
air and radiates invisible light at you to make you hot.
If we were surrounded by white objects, they would
reflect light at us and make us hotter that way. This definitely would
happen if the
objects were like mirrors, and all arranged to reflect the sun's light at us.
That
would be a solar collector, and with enough mirrors you can melt steel.
So if we were standing on a desert of white sand, we would notice that we
were
getting extra energy from the sand. But white objects reflect light in all
directions,
and not preferentially at a particular object, and they bounce a lot of the
light back
into the sky, so there is less down here. The reflected light is still
visible light, which
does not warm the air (air doesn't notice visible light. That's why we can see
through air).
The simplest way to keep sunlight from making us hot is to refuse delivery of it,
which is what a white object does.
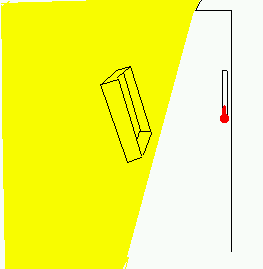
The experiment to demonstrate your effect
might be to have a thermometer
in
the shade of the house, in a place where there was sunlight nearby (the
thermometer
right at the corner of the house, or on the north side of the house close to noon) and then
compare a pair
of reflectors, one that is all white and one that is all black. We want the
reflector to
be nice and big, so that it will be redirecting a lot of energy, some at the
thermometer.
The top of the box that copier paper comes in might do -- still bigger would be
better,
but these are not easy to come by, and bigger isn't important if we can get within
a foot or two
of the thermometer and still be in the sunlight. (Another way to do this
would be
inside, late in the afternoon or early in the morning, using a sunbeam coming
in a
window to redirect with our reflector). Now I'm betting that a white object
would cause a temperature rise, and that a black one would not.
My Question:
How are thermal energy, temperature, and the kinetic energy of molecules related?
Are there equations defining their relationships?
Joe's answer
However, molecules can rotate and vibrate as well as just move, and then
the average kinetic energy can be several times larger when we include these other
ways to move.
At very low temperatures, there is a complication called quantum
mechanics, which means that atoms and molecules don't act quite the same
as marbles or baseballs; as a consequence a molecule can have a
certain amount of kinetic energy even though in a way it isn't moving at all.
(If this part doesn't make sense, ignore it.)
Thermal energy can be defined as the sum of all the energy that an object has
that is related to its temperature. Now in addition to the various
kinds of motion there is a kind of energy that comes from molecules
interacting with each other. This can be as big as the kinetic
energy. Note that when I say "thermal energy" I mean the total energy of
the object, rather than the energy per molecule or per atom or per
gram. Then the Atlantic ocean has more thermal energy than a hot cup
of coffee, just because there is more of it. But some of the time we
will talk about the energy per gram -- we should say "thermal energy
density" or "specific thermal energy" ("specific:" being an
old-fashioned way to say "per unit of mass").
The chemists have a unit called "a mole" which is 6 x 10^23
particles. One mole of ordinary materials is a about a teaspoonful
-- the unit exists so that we can translate atomic facts into
measurable numbers. Then the average kinetic energy of a mole of almost
anything is 12 Joules x the Kelvin temperature, and the thermal energy
of a mole of almost anything is larger than this by at most a factor of
3 (due to the interaction energy contribution).
My Question:
I'm doing a project to detect temperatures, using liquid crystals.
Will the crystals change color based on the heat applied?"
Joe's answer
The material has a temperature range that is set back at the factory. We use the
20-25 C (68F-77 F) and 25 C - 30 C (77 F -86 F) ranges a lot. At room temperature it
is black (unless the room is really warm); it is black at all temperatures below its active
range. Within the range it turns brown (a dull red, really) and then tan (or dull
orange-yellow), and then green, and then blue, and finally violet as it warms through
the range. Above the upper limit it remains violet, getting darker and darker. Within
the range, you can detect temperature variations (from place to place) of as little as 1/2
degree F as a slight difference in color. It is hard to tell the actual temperature, but for
comparing temperatures it works very well.
The word "heat" sometimes refers to an action (making something hot, by adding energy), and sometimes to the amount of energy in something. The liquid crystal doesn't measure those things -- just the temperature itself.
The sun is very hot, and the result is that the brightest part of
the spectrum is in the visible. It depends a little bit on how you
make the spectrum (i.e. what the prism is made of).
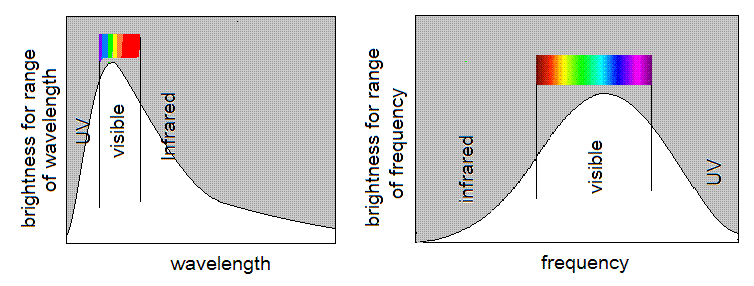 Either way, the peak turns out to be in the middle of the sun's spectrum.
Either way, the peak turns out to be in the middle of the sun's spectrum.
Under the right circumstances, it probably does. Here are the concepts that combine to have this effect:
-- to us, from the sun, in the form of visible light
-- and also away from the earth, in the form of infrared (invisible) light
*
Dark objects absorb visible light, but they also participate in the second
step, where light leaves again.
*
Light colored objects reflect light, and don't have any effect on it.
*
Adding energy to something makes it hotter, almost always.
The kinetic energy of a molecule is closely related
to temperature. For most molecules near room
temperature, the average of the part of the kinetic energy that is due
to motion of the center of mass is given to very good accuracy by KE =
3kT/2 where T is the temperature in Kelvin and k is a constant whose
value is well known (1.38 x 10^(-23)) J/Kelvin. What it means is that
the temperature is exactly the average kinetic energy except that we
measure it in units of the wrong size.
Although the material is called a "liquid crystal," what you will actually be using is a
plastic sheet that is slightly thicker than the cardstock they make file folders from. It
will flex easily and can be cut with scissors. Inside the plastic are small droplets of the
actual material, which is a liquid. The cut edges are slightly sticky, and you have to be
careful to keep it between layers of waxed paper when you are not using it (or it will
stick to something permanently).
Return to Question Board Main Page